Equipment qualification for the medical technology industry
KKS has many years of expertise in the qualification of process equipment and offers its customers the qualification of equipment as a service. Alongside system development, the entire equipment qualification process is carried out and documented by KKS during the design and implementation phase. Equipment qualification is coordinated by a KKS project manager in close collaboration with the customer on the basis of a qualification plan. Every qualification step (DQ/IQ/OQ) is concluded with a customer sign-off. KKS equipment qualification is based on the established V model.
Equipment qualification services for the medical technology industry
As a general rule, a distinction is made between the following qualification and validation activities:
Equipment qualification
Execution and documentation of equipment qualification
Computer system validation of equipment (CSV)
Computer system validation of equipment (CSV)
Execution and documentation of the computer system validation of equipment
Process validation
Support for process validation
The objective of equipment qualification for the medical technology industry
First-class and qualified process systems form the basis for successful and safe production. This is why processes, particularly in regulated industries such as the medical technology industry, are validated. Qualified production equipment are a basic prerequisite for process validation. Equipment qualification provides documented verification of suitability and demonstrates that the process system delivers the expected results with the defined accuracy, reliability, reproducibility and consistent performance. Processes must be validated in accordance with EN ISO 13485 and ISO 9001 if the resulting output cannot be verified by means of subsequent monitoring or measurements. As equipment qualification forms the basis for process validation, the qualification of process systems is essential for the medical technology industry.

The objective of computer system validation of equipment (CSV)
Automated production environments are controlled by one or more machine controllers (PLCs) or a computer system. A computer system is a network or group of several computers that are controlled individually and can access shared data and devices. Validation provides documented verification that the system corresponds to the specifications throughout the entire life cycle. During the computer system validation of equipment (CSV) of a process system, defined advanced functionalities of a computer-assisted system are tested and documented. The CSV is a documented process that reproducibly verifies that a computer-assisted system is working in accordance with requirements.
The objective of process validation
Process validation provides documented verification that a process or system reproducibly satisfies the previously specified requirements when used in practice. It therefore provides proof of long-term process stability. Process validation is typically carried out by the customer or the system operator. KKS can provide customer support for this if necessary.
Engineering, implementation and equipment qualification from a single source
The entire equipment qualification process takes place in parallel with the implementation of a system and is approved by the customer in every phase. The customer receives a complete and approved qualification documentation including design qualification, installation qualification, operational qualification and, if ordered, the computer system validation of equipment (CSV). KKS projects for process systems are generally implemented on a customer-specific basis and equipment qualification should ideally take place in parallel with the project. This is the only way to ensure that the individual qualification steps can be scheduled and executed effectively and purposefully from the start of the project.
Equipment qualification takes place in several stages and typically as follows:
Thanks to our extensive expertise in equipment qualification, we are able to offer individual and qualified solutions.
Find out here how KKS carries out equipment qualification as part of its own systems engineering and how we can also support you in process validation after your system has been commissioned. We also offer qualified system solutions for all surface treatment processes, for example for the medical technology industry.
Relationship between conformity assessment, equipment qualification and validation
In addition to customer requirements, a process system must also satisfy the regulatory requirements and standards of the conformity assessment procedure in accordance with the Machinery Directive as well as qualification and process validation.
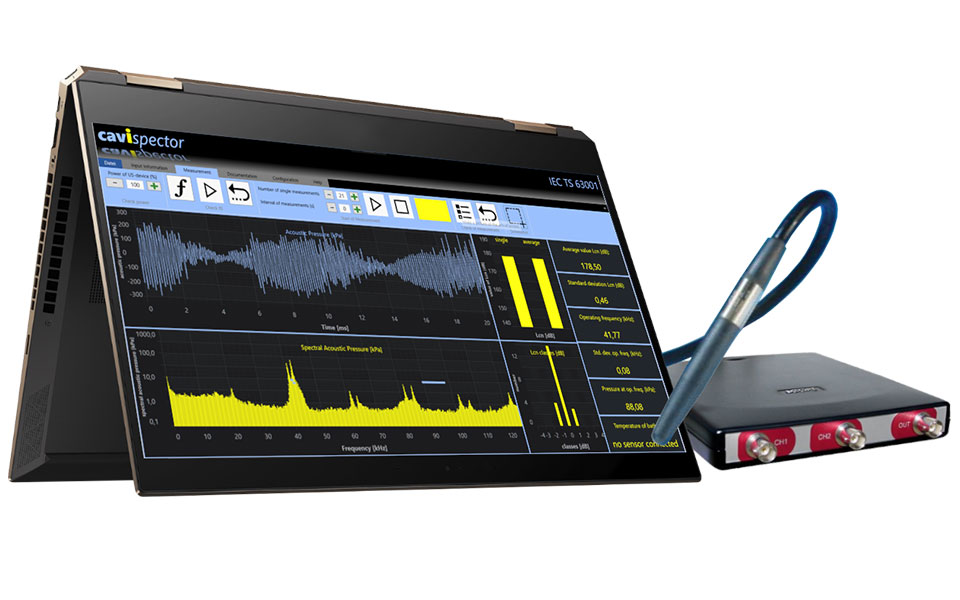
Qualification of ultrasonic technology
At KKS, ultrasonic technology solutions are qualified by means of a measurement method specified by the IEC (= International Electrotechnical Commission) under IEC TS 63001. In order to analyze the parameters that influence the cleaning effect, KKS uses the so-called Cavispector analysis software. A hydrophone is used to measure the cavitation noise level and temperature in the medium at full power and these are documented as a reference in a measurement report. The cavitation behavior of ultrasonic tanks can be regularly checked and verified as part of a service that is provided at the customer’s premises.